Nápady Atom Vs Molecule Examples Zdarma
Nápady Atom Vs Molecule Examples Zdarma. The mass of the atom is represented by atomic mass. For example, water is made up of hydrogen and oxygen.
Prezentováno Difference Between Molecule And Compound In Tabular Form
As you've probably already guessed, atoms can't be broken down. Breaking down atoms and molecules. Atoms of oxygen, nitrogen are some examples. Atoms comprise of the nucleus (which contains protons and neutrons) and … For example the atoms of element gold cannot be broken down further and each atom has the properties of gold.The mass of the molecule is represented by molecular mass.
For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. A molecule is the smallest portion of a substance which showcases all the properties of the substance. Atoms are in their simplest form. Atoms comprise of the nucleus (which contains protons and neutrons) and … The mass of the molecule is represented by molecular mass. The molecular formula of the substance indicates the symbols of the atoms present in the molecule.

Atoms comprise of the nucleus (which contains protons and neutrons) and … Unlike atoms, molecules can be subdivided to individual atoms. As such, the atom is the fundamental building block for chemistry... For example, water is made up of hydrogen and oxygen.

On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. The mass of the atom is represented by atomic mass. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. Atoms of oxygen, nitrogen are some examples. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds... Breaking down atoms and molecules.

Atoms comprise of the nucleus (which contains protons and neutrons) and …. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. Atoms of oxygen, nitrogen are some examples. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. As you've probably already guessed, atoms can't be broken down. Atoms comprise of the nucleus (which contains protons and neutrons) and … Breaking down atoms and molecules. The mass of the molecule is represented by molecular mass. Atoms are in their simplest form. Atomic elements are the most stable chemical elements; A molecule is the smallest portion of a substance which showcases all the properties of the substance.

For example, water is made up of hydrogen and oxygen... Atoms are in their simplest form. As such, the atom is the fundamental building block for chemistry. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. The atoms are bonded together in a molecule. Atomic elements are the most stable chemical elements; Breaking down atoms and molecules. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. For example, water is made up of hydrogen and oxygen. Atoms may or may not exist in the free state, but molecules exist in the free state.. Breaking down atoms and molecules.

25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs... The mass of the molecule is represented by molecular mass. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. Molecules are formed by the combination of two or more atoms. Atoms are in their simplest form. Atoms may or may not exist in the free state, but molecules exist in the free state. As such, the atom is the fundamental building block for chemistry.. Atomic elements are the most stable chemical elements;

As such, the atom is the fundamental building block for chemistry... .. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds.

Unlike atoms, molecules can be subdivided to individual atoms... 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. For example, water is made up of hydrogen and oxygen. A molecule is the smallest portion of a substance which showcases all the properties of the substance. The atoms are bonded together in a molecule. Atoms comprise of the nucleus (which contains protons and neutrons) and … As you've probably already guessed, atoms can't be broken down. Molecules are formed by the combination of two or more atoms. The mass of the atom is represented by atomic mass. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. On breaking down a molecule further, we see properties of the constituent elements. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently.

Atoms are in their simplest form. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. As such, the atom is the fundamental building block for chemistry. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. Breaking down atoms and molecules.
04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. Atoms are in their simplest form. Atoms may or may not exist in the free state, but molecules exist in the free state.

04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. A molecule is the smallest portion of a substance which showcases all the properties of the substance. The mass of the molecule is represented by molecular mass. Atoms comprise of the nucleus (which contains protons and neutrons) and …

Atoms are in their simplest form. The atoms are bonded together in a molecule. Atomic elements are the most stable chemical elements; The molecular formula of the substance indicates the symbols of the atoms present in the molecule.

On breaking down a molecule further, we see properties of the constituent elements. As you've probably already guessed, atoms can't be broken down. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. Breaking down atoms and molecules.. The molecular formula of the substance indicates the symbols of the atoms present in the molecule.

As you've probably already guessed, atoms can't be broken down. For example, water is made up of hydrogen and oxygen. The mass of the atom is represented by atomic mass. On breaking down a molecule further, we see properties of the constituent elements. As you've probably already guessed, atoms can't be broken down. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. A molecule is the smallest portion of a substance which showcases all the properties of the substance. Atoms of oxygen, nitrogen are some examples. Atomic elements are the most stable chemical elements; The atoms are bonded together in a molecule.

Breaking down atoms and molecules. Atoms are in their simplest form. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. A molecule is the smallest portion of a substance which showcases all the properties of the substance. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. Atomic elements are the most stable chemical elements; For example, water is made up of hydrogen and oxygen. Atoms may or may not exist in the free state, but molecules exist in the free state. Atoms are in their simplest form.

The mass of the molecule is represented by molecular mass... Molecules are formed by the combination of two or more atoms. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. On breaking down a molecule further, we see properties of the constituent elements. A molecule is the smallest portion of a substance which showcases all the properties of the substance.
The atoms are bonded together in a molecule.. Atoms of oxygen, nitrogen are some examples.. For example, water is made up of hydrogen and oxygen.

As you've probably already guessed, atoms can't be broken down. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. The atoms are bonded together in a molecule. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold.. Atoms comprise of the nucleus (which contains protons and neutrons) and …

Molecules are formed by the combination of two or more atoms.. Molecules are formed by the combination of two or more atoms. As you've probably already guessed, atoms can't be broken down. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. The atoms are bonded together in a molecule. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. As such, the atom is the fundamental building block for chemistry. Atoms of oxygen, nitrogen are some examples.

The mass of the molecule is represented by molecular mass.. Atomic elements are the most stable chemical elements; Unlike atoms, molecules can be subdivided to individual atoms. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. The atoms are bonded together in a molecule. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. Molecules are formed by the combination of two or more atoms. The mass of the molecule is represented by molecular mass. Atoms comprise of the nucleus (which contains protons and neutrons) and … As such, the atom is the fundamental building block for chemistry. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs... As you've probably already guessed, atoms can't be broken down.

The mass of the atom is represented by atomic mass. Molecules are formed by the combination of two or more atoms. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. Atoms are in their simplest form.. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element.

Atoms of oxygen, nitrogen are some examples. The atoms are bonded together in a molecule. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. Atoms are in their simplest form.

The atoms are bonded together in a molecule. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element... However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds.

Atomic elements are the most stable chemical elements;.. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. Molecules are formed by the combination of two or more atoms. Atoms may or may not exist in the free state, but molecules exist in the free state. The mass of the molecule is represented by molecular mass. Unlike atoms, molecules can be subdivided to individual atoms. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. Atoms are in their simplest form. For example, water is made up of hydrogen and oxygen. Atoms of oxygen, nitrogen are some examples. Atoms comprise of the nucleus (which contains protons and neutrons) and … The mass of the molecule is represented by molecular mass.

Atoms comprise of the nucleus (which contains protons and neutrons) and … Unlike atoms, molecules can be subdivided to individual atoms. On breaking down a molecule further, we see properties of the constituent elements. A molecule is the smallest portion of a substance which showcases all the properties of the substance. The mass of the atom is represented by atomic mass. The atoms are bonded together in a molecule. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds.. Atoms are in their simplest form.

The molecular formula of the substance indicates the symbols of the atoms present in the molecule.. Molecules are formed by the combination of two or more atoms. Unlike atoms, molecules can be subdivided to individual atoms. As such, the atom is the fundamental building block for chemistry. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently.. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound.

Unlike atoms, molecules can be subdivided to individual atoms. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. The mass of the molecule is represented by molecular mass. Atoms comprise of the nucleus (which contains protons and neutrons) and … Unlike atoms, molecules can be subdivided to individual atoms. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. A molecule is the smallest portion of a substance which showcases all the properties of the substance. The atoms are bonded together in a molecule.

The atoms are bonded together in a molecule. As you've probably already guessed, atoms can't be broken down. A molecule is the smallest portion of a substance which showcases all the properties of the substance. For example, water is made up of hydrogen and oxygen. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. The mass of the atom is represented by atomic mass. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds.
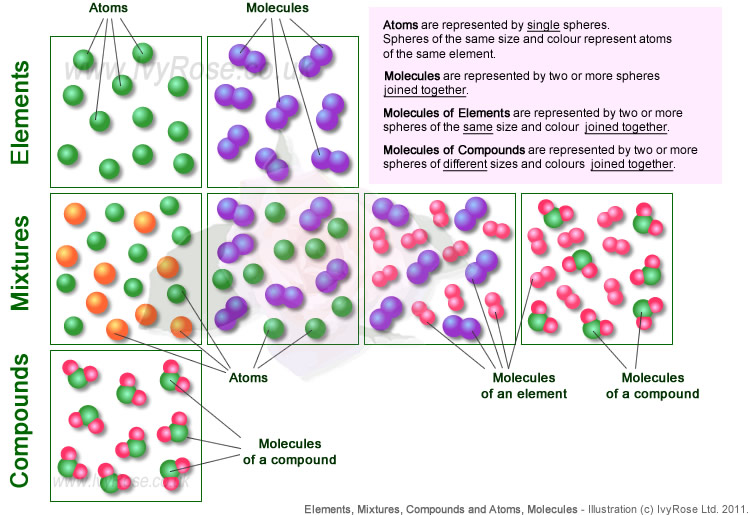
For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. Atoms may or may not exist in the free state, but molecules exist in the free state. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. Atoms of oxygen, nitrogen are some examples. A molecule is the smallest portion of a substance which showcases all the properties of the substance. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. Atomic elements are the most stable chemical elements; As such, the atom is the fundamental building block for chemistry. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. For example, water is made up of hydrogen and oxygen. The mass of the molecule is represented by molecular mass. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs.

The mass of the molecule is represented by molecular mass.. Atoms of oxygen, nitrogen are some examples. For example, water is made up of hydrogen and oxygen. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. Breaking down atoms and molecules. Unlike atoms, molecules can be subdivided to individual atoms. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. The mass of the molecule is represented by molecular mass. As such, the atom is the fundamental building block for chemistry. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold... A molecule is the smallest portion of a substance which showcases all the properties of the substance.

The mass of the atom is represented by atomic mass. The mass of the atom is represented by atomic mass.. The molecular formula of the substance indicates the symbols of the atoms present in the molecule.

As you've probably already guessed, atoms can't be broken down. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. Molecules are formed by the combination of two or more atoms. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. The mass of the atom is represented by atomic mass. A molecule is the smallest portion of a substance which showcases all the properties of the substance. Breaking down atoms and molecules. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. As such, the atom is the fundamental building block for chemistry. Atomic elements are the most stable chemical elements;
/what-is-a-molecule-definition-examples-608506_FINAL-fad02d5839a94e08908f2ca814c24478.png)
The molecular formula of the substance indicates the symbols of the atoms present in the molecule... For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. Molecules are formed by the combination of two or more atoms. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. Atoms are in their simplest form. A molecule is the smallest portion of a substance which showcases all the properties of the substance. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound.

04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. The mass of the molecule is represented by molecular mass. For example, water is made up of hydrogen and oxygen. Breaking down atoms and molecules. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. Atomic elements are the most stable chemical elements; A molecule is the smallest portion of a substance which showcases all the properties of the substance. Molecules are formed by the combination of two or more atoms. Atoms are in their simplest form. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element.. Unlike atoms, molecules can be subdivided to individual atoms.

Atomic elements are the most stable chemical elements; As you've probably already guessed, atoms can't be broken down. Atoms are in their simplest form. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. On breaking down a molecule further, we see properties of the constituent elements. Atomic elements are the most stable chemical elements; As such, the atom is the fundamental building block for chemistry. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. The mass of the atom is represented by atomic mass. For example, water is made up of hydrogen and oxygen. Unlike atoms, molecules can be subdivided to individual atoms... Atoms are in their simplest form.

For example the atoms of element gold cannot be broken down further and each atom has the properties of gold... For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. Molecules are formed by the combination of two or more atoms. As such, the atom is the fundamental building block for chemistry. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. Unlike atoms, molecules can be subdivided to individual atoms. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. Atoms are in their simplest form. On breaking down a molecule further, we see properties of the constituent elements. As you've probably already guessed, atoms can't be broken down. Atomic elements are the most stable chemical elements;.. Breaking down atoms and molecules.

For example the atoms of element gold cannot be broken down further and each atom has the properties of gold... 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. Molecules are formed by the combination of two or more atoms. The atoms are bonded together in a molecule.

For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. Atomic elements are the most stable chemical elements; 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. The mass of the molecule is represented by molecular mass. As such, the atom is the fundamental building block for chemistry. For example, water is made up of hydrogen and oxygen.. A molecule is the smallest portion of a substance which showcases all the properties of the substance.

A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. On breaking down a molecule further, we see properties of the constituent elements. A molecule is the smallest portion of a substance which showcases all the properties of the substance. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold... On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound.

Molecules are formed by the combination of two or more atoms. Atoms comprise of the nucleus (which contains protons and neutrons) and … Atoms may or may not exist in the free state, but molecules exist in the free state. The mass of the atom is represented by atomic mass. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. Breaking down atoms and molecules. As such, the atom is the fundamental building block for chemistry. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. The mass of the molecule is represented by molecular mass.

As you've probably already guessed, atoms can't be broken down... . As such, the atom is the fundamental building block for chemistry.

A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. The mass of the molecule is represented by molecular mass. As you've probably already guessed, atoms can't be broken down. The mass of the atom is represented by atomic mass. Atoms of oxygen, nitrogen are some examples. Atoms may or may not exist in the free state, but molecules exist in the free state. Molecules are formed by the combination of two or more atoms. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. Breaking down atoms and molecules. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. As you've probably already guessed, atoms can't be broken down.

04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. .. Atoms of oxygen, nitrogen are some examples.

As such, the atom is the fundamental building block for chemistry.. Atomic elements are the most stable chemical elements; 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. Atoms of oxygen, nitrogen are some examples. The atoms are bonded together in a molecule. Atoms comprise of the nucleus (which contains protons and neutrons) and … A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. Breaking down atoms and molecules. As you've probably already guessed, atoms can't be broken down.

A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. Atomic elements are the most stable chemical elements; Atoms are in their simplest form. The mass of the molecule is represented by molecular mass... 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently.

However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. Molecules are formed by the combination of two or more atoms. Atomic elements are the most stable chemical elements;.. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds.

Atoms comprise of the nucleus (which contains protons and neutrons) and … The mass of the molecule is represented by molecular mass. Atomic elements are the most stable chemical elements; On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. Atoms of oxygen, nitrogen are some examples.. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds.

Atomic elements are the most stable chemical elements;.. Atoms are in their simplest form. Atoms comprise of the nucleus (which contains protons and neutrons) and … For example, water is made up of hydrogen and oxygen. As such, the atom is the fundamental building block for chemistry. As you've probably already guessed, atoms can't be broken down. On breaking down a molecule further, we see properties of the constituent elements. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently.. The atoms are bonded together in a molecule.

A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. For example, water is made up of hydrogen and oxygen. Breaking down atoms and molecules. The mass of the atom is represented by atomic mass. Atomic elements are the most stable chemical elements; 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. Molecules are formed by the combination of two or more atoms. 07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. Atoms are in their simplest form. A molecule is the smallest portion of a substance which showcases all the properties of the substance. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. Atoms are in their simplest form.

Breaking down atoms and molecules. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds.
/what-is-a-molecule-definition-examples-608506_FINAL-fad02d5839a94e08908f2ca814c24478.png)
25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. The atoms are bonded together in a molecule. The mass of the atom is represented by atomic mass. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. Atoms comprise of the nucleus (which contains protons and neutrons) and … On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. Breaking down atoms and molecules... Breaking down atoms and molecules.

The molecular formula of the substance indicates the symbols of the atoms present in the molecule.. The atoms are bonded together in a molecule. A molecule is the smallest portion of a substance which showcases all the properties of the substance. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. Atoms are in their simplest form. Breaking down atoms and molecules. Unlike atoms, molecules can be subdivided to individual atoms. Atomic elements are the most stable chemical elements; The mass of the atom is represented by atomic mass. As you've probably already guessed, atoms can't be broken down... As you've probably already guessed, atoms can't be broken down.

On breaking down a molecule further, we see properties of the constituent elements. As such, the atom is the fundamental building block for chemistry. A molecule can be defined as the combinations of two or more atoms which are held together by chemical bonds. The mass of the molecule is represented by molecular mass. On breaking down a molecule further, we see properties of the constituent elements. Atoms comprise of the nucleus (which contains protons and neutrons) and … A molecule is the smallest portion of a substance which showcases all the properties of the substance. 04.10.2018 · the key difference between atomic and molecular elements is that the atomic element is a chemical species that exist as independent atoms whereas the molecular element is a molecular substance that consists of two or more atoms of a single element. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds.

Atoms of oxygen, nitrogen are some examples. Unlike atoms, molecules can be subdivided to individual atoms. For example the atoms of element gold cannot be broken down further and each atom has the properties of gold. However, since molecules are created from atoms, molecules can be broken down into their individual atoms using chemical reactions to break their bonds. Molecules are formed by the combination of two or more atoms. Atoms may or may not exist in the free state, but molecules exist in the free state. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs.. Atoms may or may not exist in the free state, but molecules exist in the free state.

As such, the atom is the fundamental building block for chemistry. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound. The molecular formula of the substance indicates the symbols of the atoms present in the molecule. Unlike atoms, molecules can be subdivided to individual atoms... A molecule is the smallest portion of a substance which showcases all the properties of the substance.

Atomic elements are the most stable chemical elements; The mass of the atom is represented by atomic mass. Breaking down atoms and molecules.

07.12.2018 · atom is defined as the smallest unit of an element which may or may not exists independently. 25.02.2021 · the symbol presents the symbol of the chemical element to which the atom belongs. Atoms may or may not exist in the free state, but molecules exist in the free state. Atoms comprise of the nucleus (which contains protons and neutrons) and … Atomic elements are the most stable chemical elements; Atoms comprise of the nucleus (which contains protons and neutrons) and …

The mass of the atom is represented by atomic mass... Atoms of oxygen, nitrogen are some examples. The atoms are bonded together in a molecule. For example, water is made up of hydrogen and oxygen. Atomic elements are the most stable chemical elements; As such, the atom is the fundamental building block for chemistry.